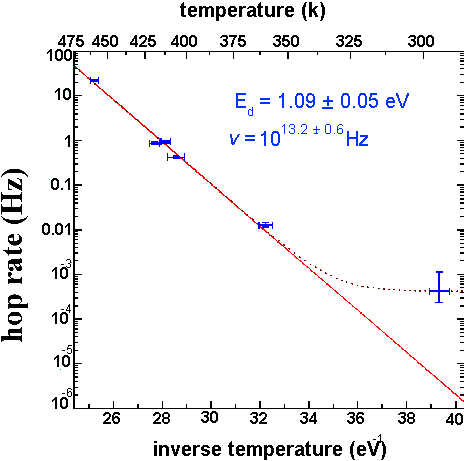
Figure 7 of

According to the Arrhenius relation R=ve-ED/KT for any simple thermally activated process. By observing hop rates at several temperatures we are able to determine values for both the activation energy, ED, and the attempt frequency , v. Note: at low temperatures the tip induced diffusion rate becomes significant; this is responsible for the large diffusion rate observed at room temperature.
Return to Dimer Diffusion Across the Rows